Introduction #
PUREX (Plutonium Uranium Extraction) is a chemical process used to extract plutonium and uranium from spent fuel in nuclear reactors for the production of Mixed Oxide Fuel (MOX) fuel that can be reused in a reactor. The remaining fission products and transuranics, which are actually waste, now take up much less space and can therefore be disposed of much more cheaply [1].
The PUREX method is one of the most widely used methods. It is used at the Orano plant in La Hague in France. Likewise in Japan and Russia.
Steps in the PUREX process #
Division process (shearing) #
First, the end pieces of the fuel elements are removed, as they may contain more radioactive materials and have a higher heat development.
After removing the end pieces, the rest of the fuel elements are cut into smaller pieces with a length of typically 35 millimeters. This division into smaller pieces facilitates later steps in the reprocessing process, where the extraction and processing of the recyclable materials, such as uranium and plutonium, occurs.

Leaching #
The split pieces of fuel are placed in a solvent, usually a strong oxidizer such as nitric acid (HNO3). The solvent reacts with the fuel and dissolves the desired materials, such as plutonium and uranium, forming a solution called “fuel solution
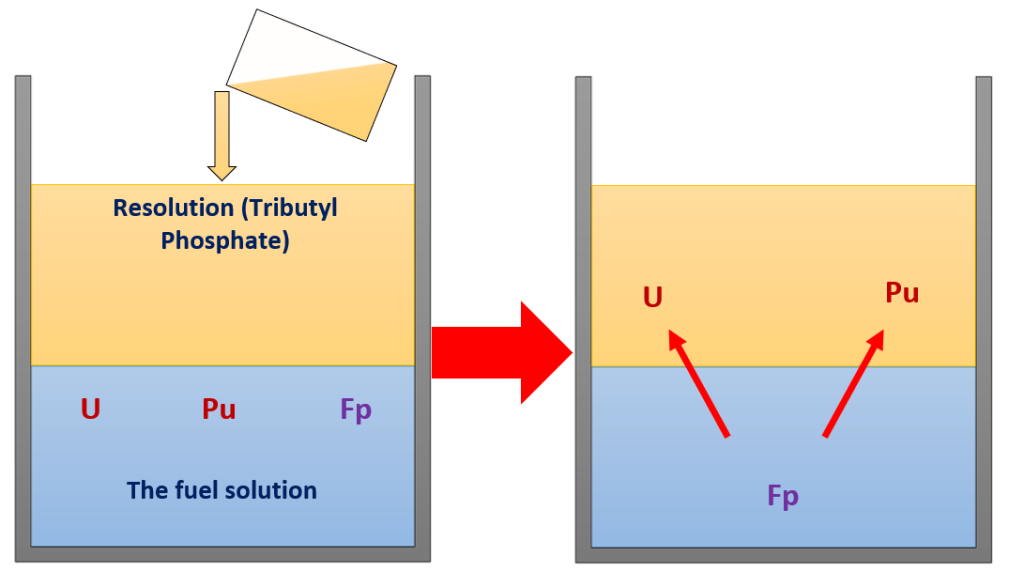
The solution contains recyclable uranium (U), plutonium (Pu) and non-recyclable fission products (Fp) which are actually waste.

Extraction – separating uranium and plutonium from the fission products #
The next step is to separate uranium and plutonium from the fission products. This is achieved by mixing the solution with a chemical called tributyl phosphate (TBP) [2]. When the solution is mixed with TBP, stable complex compounds are formed whereby uranium (U) and plutonium (Pu) are absorbed and separated from the fission products (Fp)
Stripping – separation of uranium and plutonium #
The stripping step involves separating uranium from plutonium. This is done using a combination of nitric acid (HNO3) and a reducing agent such as hydrazine (N2H4) [3].
The nitric acid frees the uranium and plutonium from the organic solvents used in the extraction phase. While hydrazine (N2H4) is used to reduce the uranium to a lower oxidation state, leading to the formation of solid uranium while the plutonium remains in solution.
The result is a separated phase where the uranium is in the form of uranium nitrate complexes and the plutonium remains in the reduced extractant.

The end product from PUREX #

Recyclable materials #
After going through a PUREX process, up to 95% of the spent nuclear fuel can be recycled to produce new fuel, so-called Mixed Oxide Fuel (MOX) fuel. It is most often in a mixture ratio of 70% recycled MOX and 30% new fuel.
Non-recyclable materials #
After the PUREX process, the non-recyclable materials make up about 5% of the original used fuel. These include fission products, actinides.
However, the upcoming Generation IV reactor technologies can recycle the actinides.
Fission products for medical purposes. #
Some of the fission products separated in PUREX may have useful properties. There may be attempts to develop technologies to utilize certain fission products for medical purposes.
An example is technetium-99m (Tc-99m). It is one of the most widely used isotopes in nuclear medicine for diagnostic purposes. It is usually generated by irradiating natural uranium-235 (U-235) in a fission reactor and then performing a chemical process to separate and purify the Tc-99m from the rest of the fission products [4].
Another known fission isotope with potential medical applications is iodine-131 (I-131). This radioactive isotope can be used in the treatment of thyroid diseases, such as hyperthyroidism and certain types of thyroid cancer. I-131 emits beta radiation that can destroy thyroid tissue, including cancer cells [5].
PUREX does not increase proliferation #
The PUREX process does not create weapons-grade plutonium (Pu). Although the method extracts plutonium, it will still contain a mixture of isotopes, including Pu-239, Pu-240 and other plutonium isotopes. To produce weapons-grade plutonium with high purity and low content of unwanted isotopes such as Pu-240, additional steps are needed, such as chemical purification and concentration.
To produce plutonium for use in weapons programs, a more advanced and specialized process called isotope separation is required. This involves several steps of chemical treatment, purification and concentration to obtain plutonium with a very high concentration of Pu-239 and a low content of other isotopes.
This is not involved in the reprocessing and recycling of spent reactor fuel.
Read more about why nuclear energy does not produce more nuclear weapons
Sources #
- Processing of Used Nuclear Fuel – World Nuclear Association (world-nuclear.org)
- Tributyl phosphate – Wikipedia
- Technetium – Wikipedia
- Solvent-extraction complexes of the uranyl ion. 2. Crystal and molecular structures of catena-bis(.mu.-di-n-butyl phosphato-O,O’)dioxouranium(VI) and bis(.mu.-di-n-butyl phosphato-O,O’)bis[(nitrato)(tri-n-butylphosphine oxide)dioxouranium(VI)] | Inorganic Chemistry (acs.org)
- Iodine-131 – Wikipedia




